AURAMINE O
Synonyms: PYOCTANINUMAUREUM;PYOCTANUNUMAUREUM;PYOKTANINYELLOW;PYKOTANNIN;AURAMINEO,ChemicalbookCERTIFIED;AURAMINEO,CERTIFIED(C.I.41000);AURAMINEO,FORMICROSCOPY;BASICYELLOW2.
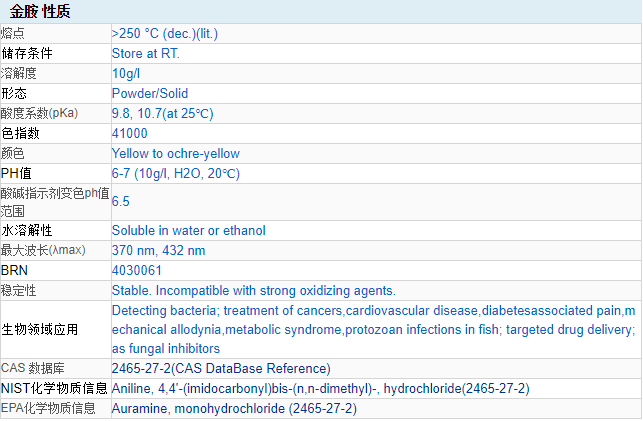
CAS Number: 2465-27-2
Molecular formula: C17H22ClN3
Molecular weight: 303.83
EINECS number: 219-567-2
Related categories: other biochemical reagents; dyes and dyes; food colorings; pigments; biochemical reagents; gold-containing catalysts; food colorings; dyes; cationic dyes; general basic dyes; hematology and histology; imprinting and staining agents; Paints and coatings; reference materials; organic Chemicalbook chemical raw materials; chemical products-inorganic chemicals; chemical products-organic chemicals; biochemical reagents-pigments; chemicals; inorganic salts; chemical materials; Dyes and Pigments; Organics; Diphenylmethane
Auramine Usage and synthesis method:
Chemical properties Yellow uniform powder. It is soluble in cold water, easily soluble in hot water, it is bright yellow, and it will decompose after boiling. It is yellow when soluble in ethanol. The dye powder is colorless in concentrated sulfuric acid, and turns light yellow after dilution; orange in concentrated nitric acid; white precipitate in sodium hydroxide solution.
Uses:
1)Basic bright yellow O can be used for dyeing silk, cotton, acrylic fiber, wool, etc., and also for direct printing. When using, the dissolving temperature should not exceed 60°C. Because of its poor light fastness, it has been seldom used in textiles. It can be used for coloring leather, paper, paint, etc.
2)Used for cellulose acetate, mordant cotton, but low fastness, bright color, can be used to make green or red, etc. It can also be used for dyeing leather, paper, linen and viscose. Alkaline can be used to color oil, fat, paint, etc. Color lakes can also be prepared for use in inks.
3)Mainly used for fluorescent staining of acid-resistant bacteria such as Mycobacterium tuberculosis. After staining with the fluorescent dye AuramineO, the acid-fast bacteria will emit a bright orange color when inspected with a fluorescent microscope containing a Chemicalbook ultraviolet light source. This method can be used for lower magnification microscopy, so acid-resistant bacteria can be found more quickly.
Production method: N,N-dimethylaniline and formaldehyde are condensed, after distillation, crystallization and purification, ammoniated with sulfur, urea and ammonium chloride, then filtered and dried to obtain the finished product. Raw material consumption (kg/t Chemicalbook) N,N-dimethylaniline (98%) 110 formaldehyde (37%) 460 urea 700 sulfur (99%) 350 ammonium chloride 630 p-aminobenzene sulfonic acid (100%) 8 refined salt 7500.
Method 1: The sintering method uses N,N-dimethylaniline as the main raw material. First, it is condensed with formaldehyde to obtain diarylmethane. After distillation, crystallization and purification, it is ammoniated with urea, sulfur, and ammonium chloride, and then filtered, The finished product is obtained after drying. . The amination reaction is actually a three-step reaction of vulcanization, imination and salt formation in one step, that is, 4,4′-dimethylaminodiphenylmethane, sulfur, urea and ammonium chloride are added to the amination kettle in proportion, and the temperature is increased to (200 ±5)℃, react for 4h, and get it out of Chemicalbook. Method 2: Solvent method The newly developed solvent method uses ethylene glycol as the solvent to reduce the reaction temperature and greatly increase the yield. The reaction process is as follows: Put 300g of ethylene glycol and 58g of sulfur into the reaction kettle, and pass in ammonia gas at (140±5)℃, add 80g of ammonium chloride after 4 hours of reaction, continue the reaction of ammonia gas for 16 hours, and the total amount of ammonia gas is about 102g. After the reaction is completed, cooling, crystallization, filtering, and drying, the product is about 155g.
Post time: Apr-29-2021





